Redox reactions, formally known as reduction-oxidation reactions, encompass a broad category of chemical reactions that involve a change in the oxidation state of atoms participating in the reaction. These changes can manifest as either straightforward processes—like the conversion of carbon into carbon dioxide (CO2) when it undergoes oxidation, or the transformation of carbon into methane (CH4) when it is reduced by hydrogen—or as multifaceted mechanisms, such as the step-by-step electron transfer reactions occurring when glucose (C6H12O6) is metabolized in the human body.
Fundamentals of Oxidation States
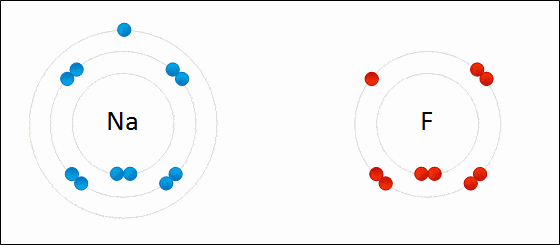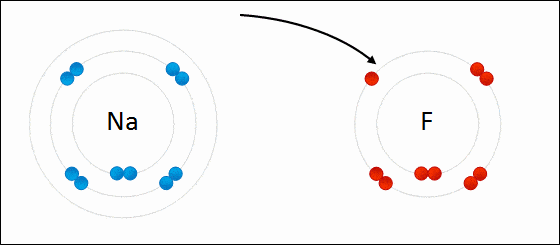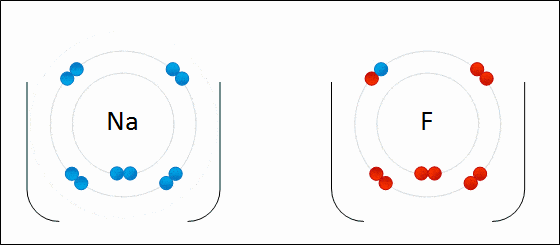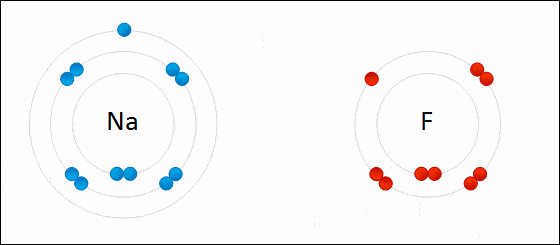
Before diving into the details of redox reactions, it is crucial to understand the concept of oxidation states. An oxidation state is a numerical value assigned to an atom in a chemical compound, and it helps in determining the extent to which the atom has been oxidized or reduced. Generally speaking, an increase in the oxidation state indicates oxidation, while a decrease represents reduction.
Types of Redox Reactions
Redox reactions can be classified into different types depending on various factors like the number of reactants and products, whether the reaction is spontaneous, and so forth. Some common types of redox reactions are:
Categories of Redox Reactions
- Combustion Reactions: These involve a fuel, often carbon-based, reacting with an oxidizer, commonly oxygen, to produce heat and light.
- Disproportionation Reactions: One element undergoes both oxidation and reduction.
- Single Replacement Reactions: An element in a compound is replaced by another element.
- Double Replacement Reactions: Elements in two different compounds swap places.
Importance in Biological Systems
Redox reactions have a particular importance in biological systems. For example, cellular respiration, the process by which cells derive energy, is a series of redox reactions. Another example would be the transfer of electrons during photosynthesis in plants.
Redox Reactions in Everyday Life
These reactions are not just confined to test tubes and biology textbooks. They have practical applications in daily life as well. For instance, the rusting of iron is a redox reaction where iron reacts with water and oxygen to form iron oxide. Another example is the process of digestion in humans, which includes a series of redox reactions to break down food.
The Role of Electron Transfer
One of the fundamental aspects of a redox reaction is electron transfer. The atom that loses an electron or electrons is said to be oxidized, while the one gaining electrons is said to be reduced. This electron transfer can be a simple one-step process or can occur through multiple steps, particularly in biological systems.
Mechanism of Redox Reactions
A redox reaction generally comprises two half-reactions:
- Oxidation half-reaction: This involves the loss of electrons from a species.
- Reduction half-reaction: This involves the gain of electrons by a species.
Each half-reaction is essential for the other to occur, and they happen simultaneously. The total charge before and after the reaction remains conserved.
Redox Reactions and Energy Transfer
The conversion of energy is another noteworthy aspect of redox reactions. Often, these reactions are exothermic, meaning they release energy, usually in the form of heat. This is particularly true for combustion reactions, which are fundamental to various industries and even in domestic settings for heating and cooking.
Balancing Redox Reactions
Balancing redox reactions is critical for understanding the stoichiometry of the reaction, which in turn is essential for applications in industries and research. Various methods are available for balancing redox reactions, including the ion-electron method and the oxidation number method.
Reagents in Redox Reactions
Typical reagents that act as oxidizing or reducing agents include oxygen, hydrogen, and halogens. Oxygen is generally an excellent oxidizing agent because of its ability to accept electrons readily. Conversely, hydrogen often acts as a reducing agent, donating electrons to other species.
Role in Environmental Processes
Redox reactions play a significant role in environmental processes as well. They are critical in the cycling of elements like carbon, nitrogen, and sulfur. For example, the conversion of atmospheric nitrogen to ammonia through the nitrogen cycle involves redox reactions.
In summary, redox reactions are a broad class of chemical reactions involving the change in oxidation states of the participating atoms. These reactions are not only crucial in various scientific disciplines but also have practical applications in everyday life and environmental processes. Understanding the fundamental aspects like oxidation states, electron transfer mechanisms, energy transfer, and the role of reagents can provide a comprehensive view of these vital chemical processes.
Understanding Redox Reactions
Redox reactions, formally known as reduction-oxidation reactions, encompass a broad category of chemical reactions that involve a change in the oxidation state of atoms participating in the reaction. These changes can manifest as either straightforward processes—like the conversion of carbon into carbon dioxide (CO2) when it undergoes oxidation, or the transformation of carbon into methane (CH4) when it is reduced by hydrogen—or as multifaceted mechanisms, such as the step-by-step electron transfer reactions occurring when glucose (C6H12O6) is metabolized in the human body.
Fundamentals of Oxidation States
Before diving into the details of redox reactions, it is crucial to understand the concept of oxidation states. An oxidation state is a numerical value assigned to an atom in a chemical compound, and it helps in determining the extent to which the atom has been oxidized or reduced. Generally speaking, an increase in the oxidation state indicates oxidation, while a decrease represents reduction.
Types of Redox Reactions
Redox reactions can be classified into different types depending on various factors like the number of reactants and products, whether the reaction is spontaneous, and so forth. Some common types of redox reactions are:
Categories of Redox Reactions
- Combustion Reactions: These involve a fuel, often carbon-based, reacting with an oxidizer, commonly oxygen, to produce heat and light.
- Disproportionation Reactions: One element undergoes both oxidation and reduction.
- Single Replacement Reactions: An element in a compound is replaced by another element.
- Double Replacement Reactions: Elements in two different compounds swap places.
Importance in Biological Systems
Redox reactions have a particular importance in biological systems. For example, cellular respiration, the process by which cells derive energy, is a series of redox reactions. Another example would be the transfer of electrons during photosynthesis in plants.
Redox Reactions in Everyday Life
These reactions are not just confined to test tubes and biology textbooks. They have practical applications in daily life as well. For instance, the rusting of iron is a redox reaction where iron reacts with water and oxygen to form iron oxide. Another example is the process of digestion in humans, which includes a series of redox reactions to break down food.
The Role of Electron Transfer
One of the fundamental aspects of a redox reaction is electron transfer. The atom that loses an electron or electrons is said to be oxidized, while the one gaining electrons is said to be reduced. This electron transfer can be a simple one-step process or can occur through multiple steps, particularly in biological systems.
Mechanism of Redox Reactions
A redox reaction generally comprises two half-reactions:
- Oxidation half-reaction: This involves the loss of electrons from a species.
- Reduction half-reaction: This involves the gain of electrons by a species.
Each half-reaction is essential for the other to occur, and they happen simultaneously. The total charge before and after the reaction remains conserved.
Redox Reactions and Energy Transfer
The conversion of energy is another noteworthy aspect of redox reactions. Often, these reactions are exothermic, meaning they release energy, usually in the form of heat. This is particularly true for combustion reactions, which are fundamental to various industries and even in domestic settings for heating and cooking.
Balancing Redox Reactions
Balancing redox reactions is critical for understanding the stoichiometry of the reaction, which in turn is essential for applications in industries and research. Various methods are available for balancing redox reactions, including the ion-electron method and the oxidation number method.
Reagents in Redox Reactions
Typical reagents that act as oxidizing or reducing agents include oxygen, hydrogen, and halogens. Oxygen is generally an excellent oxidizing agent because of its ability to accept electrons readily. Conversely, hydrogen often acts as a reducing agent, donating electrons to other species.
Role in Environmental Processes
Redox reactions play a significant role in environmental processes as well. They are critical in the cycling of elements like carbon, nitrogen, and sulfur. For example, the conversion of atmospheric nitrogen to ammonia through the nitrogen cycle involves redox reactions.
In summary, redox reactions are a broad class of chemical reactions involving the change in oxidation states of the participating atoms. These reactions are not only crucial in various scientific disciplines but also have practical applications in everyday life and environmental processes. Understanding the fundamental aspects like oxidation states, electron transfer mechanisms, energy transfer, and the role of reagents can provide a comprehensive view of these vital chemical processes.